Heat, a fundamental form of energy, permeates our world, shaping its very fabric. From the sun’s scorching rays to the cozy warmth of a fire, heat exerts a profound influence on our planet, our lives, and the universe at large.
In this comprehensive exploration, we delve into the scientific definition of heat, its measurement, and its significance in thermodynamics. We unravel the intricacies of heat transfer, exploring the processes of conduction, convection, and radiation. Moreover, we examine the practical applications of heat in daily life and industry, as well as its impact on climate change and human health.
Scientific Definition of Heat
Heat is a form of energy that flows from a hotter object to a colder object. It is the energy that is transferred due to a difference in temperature.
Heat is measured in units of calories or Joules. A calorie is the amount of heat required to raise the temperature of one gram of water by one degree Celsius. A Joule is the SI unit of energy, and it is equal to the amount of work done when a force of one Newton is applied over a distance of one meter.
Heat and Temperature
Heat and temperature are often confused, but they are not the same thing. Temperature is a measure of the average kinetic energy of the particles in an object. Heat is the flow of energy from one object to another due to a difference in temperature.
Types of Heat Transfer
Heat transfer is the movement of thermal energy from one object to another. It can occur through three primary modes: conduction, convection, and radiation.
Conduction
Conduction is the transfer of heat through direct contact between two objects. It occurs when molecules in one object collide with molecules in another object, transferring their thermal energy. Metals are good conductors of heat, while materials like wood and plastic are poor conductors.Examples
of conduction include:
- Heat flowing from a hot stovetop to a pan placed on it
- Heat moving through a metal spoon placed in a hot cup of coffee
- Heat traveling through the walls of a building from the inside to the outside
Convection
Convection is the transfer of heat through the movement of fluids (liquids or gases). When a fluid is heated, it becomes less dense and rises, while cooler fluid takes its place. This creates a convection current that carries heat from one area to another.Examples
of convection include:
- Heat rising from a hot radiator and warming a room
- Heat circulating in a pot of boiling water
- Heat flowing through the Earth’s atmosphere due to the movement of air masses
Radiation
Radiation is the transfer of heat through electromagnetic waves. All objects emit thermal radiation, but the amount and wavelength of the radiation depend on the object’s temperature. Heat from the sun is an example of thermal radiation.Examples of radiation include:
- Heat radiating from a hot object, such as a fire or a light bulb
- Heat traveling from the sun to the Earth’s surface
- Heat emitted from a person’s body
Heat and Thermodynamics
Heat plays a crucial role in thermodynamics, the branch of physics that deals with the relationships between heat and other forms of energy.
Thermodynamics is governed by four fundamental laws:
- The zeroth law of thermodynamics states that if two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.
- The first law of thermodynamics, also known as the law of conservation of energy, states that the total energy of an isolated system remains constant.
- The second law of thermodynamics states that the entropy of an isolated system always increases over time.
- The third law of thermodynamics states that the entropy of a perfect crystal at absolute zero is zero.
Entropy
Entropy is a measure of the disorder of a system. The second law of thermodynamics implies that the entropy of an isolated system always increases over time. This means that isolated systems tend to become more disordered over time.
Heat can increase the entropy of a system by increasing the disorder of the system’s molecules.
Former NFL linebacker Jeremiah Trotter has been inducted into the College Football Hall of Fame. Trotter played college football at Penn State University and was a member of the Philadelphia Eagles’ Super Bowl-winning team in 2005.
Applications of Heat
Heat has numerous applications in daily life and industry, playing a crucial role in various processes.
Energy Production
Heat is a primary source of energy generation. Power plants use heat to convert water into steam, which drives turbines to produce electricity. Geothermal energy utilizes the heat from the Earth’s interior to generate electricity or heat homes.
Cooking
Heat is essential for cooking. It transforms raw ingredients into palatable meals by breaking down complex molecules and enhancing flavors. Cooking methods like boiling, frying, baking, and roasting rely on heat to achieve desired textures and tastes.
Wide receiver Brenden Rice is expected to be a top prospect in the 2024 NFL Draft . The son of former NFL star Jerry Rice, Brenden has already received a scholarship offer from Man City .
Manufacturing
Heat is used extensively in manufacturing processes. It helps shape metals, mold plastics, and produce glass. Forging, casting, and welding involve the application of heat to manipulate materials and create desired products.
Veteran journalist Dan Rather has announced his retirement after a 60-year career in broadcasting. Rather is known for his work as an anchor for CBS News and ABC News, and he has received numerous awards for his reporting.
Heat and Climate Change
Heat plays a significant role in shaping the Earth’s climate and its long-term changes. Understanding the relationship between heat and climate change is crucial for mitigating its impacts and adapting to its consequences.
Greenhouse Effect
The greenhouse effect is a natural phenomenon that contributes to global warming. Certain gases, such as carbon dioxide and methane, in the Earth’s atmosphere act as a “greenhouse,” allowing sunlight to pass through but trapping heat radiated from the Earth’s surface.
This leads to a gradual increase in global temperatures over time.
Human activities, particularly the burning of fossil fuels, have significantly increased the concentration of greenhouse gases in the atmosphere. This has intensified the greenhouse effect, leading to an accelerated rise in global temperatures and the associated impacts of climate change.
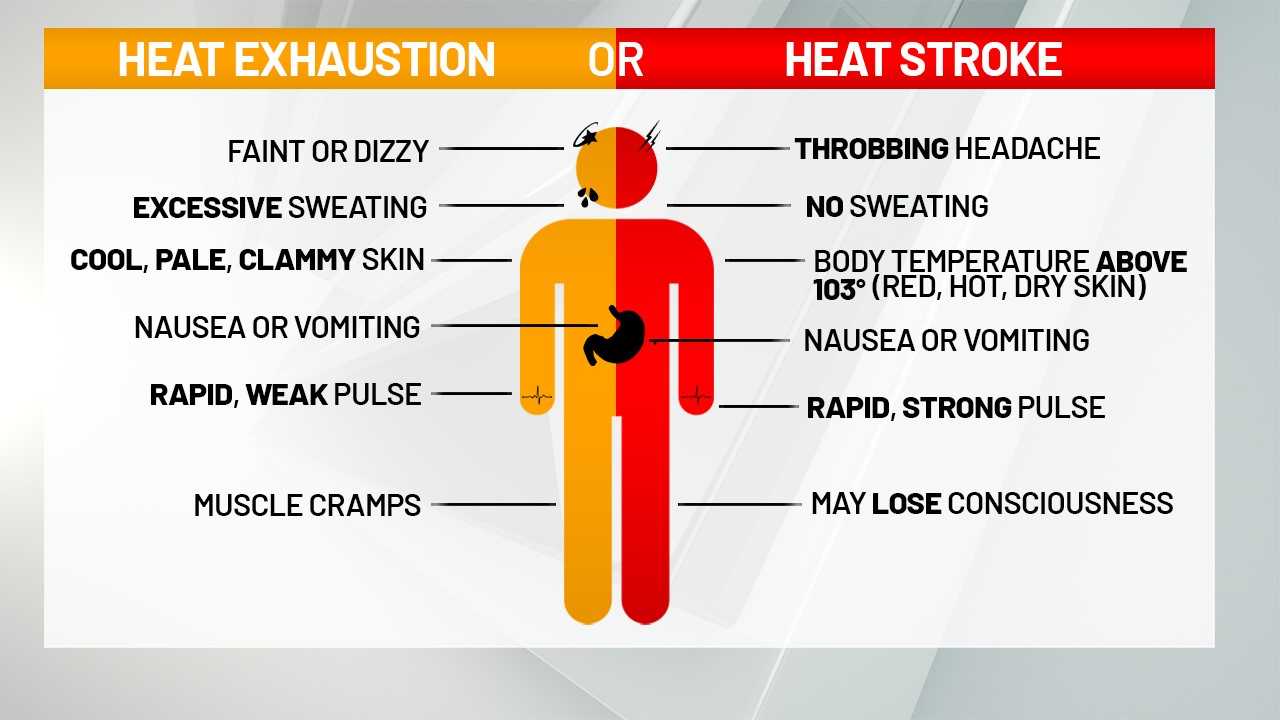
Heat and Human Health
 [/caption]
[/caption]
Heat, a form of energy, has significant effects on the human body. Exposure to extreme heat can lead to heat-related illnesses, including heatstroke and hypothermia.
Heat-Related Illnesses
Heatstrokeoccurs when the body’s core temperature rises to dangerous levels, often due to prolonged exposure to high temperatures or strenuous activity in hot environments. Symptoms include confusion, nausea, vomiting, rapid heartbeat, and loss of consciousness. Heatstroke is a medical emergency and requires immediate medical attention.
Hypothermia, on the other hand, occurs when the body loses heat faster than it can produce it. This can happen in cold environments or during prolonged immersion in cold water. Symptoms include shivering, weakness, confusion, and loss of coordination. Hypothermia can also be life-threatening and requires prompt medical attention.
Prevention and Treatment, Heat
To prevent heat-related illnesses, it is important to:
- Stay hydrated by drinking plenty of fluids, especially water or electrolyte-rich beverages.
- Avoid prolonged exposure to extreme heat, especially during the hottest hours of the day.
- Wear loose-fitting, light-colored clothing made of breathable fabrics.
- Take breaks in cool, shaded areas when working or exercising in hot environments.
If someone experiences symptoms of heatstroke or hypothermia, it is crucial to seek medical attention immediately. Treatment for heatstroke typically involves cooling the body down with cold water or ice packs and administering fluids. Hypothermia treatment involves warming the body gradually with warm blankets or clothing and providing warm fluids.
Heat and Engineering
In engineering, understanding and harnessing heat transfer is crucial for designing and optimizing various systems and devices. Heat exchangers, for instance, are essential components in numerous industrial processes, such as power generation, refrigeration, and chemical manufacturing.
The principles of heat transfer, including conduction, convection, and radiation, play a significant role in engineering applications. Engineers must carefully consider these mechanisms to ensure efficient and effective heat management in their designs.
Design of Heat Exchangers
Heat exchangers are devices designed to transfer heat between two fluids at different temperatures. They find widespread use in various industries, including power plants, chemical processing, and HVAC systems.
The design of heat exchangers involves several key considerations, such as the selection of appropriate materials, optimization of heat transfer surface area, and minimization of pressure drop. Engineers must carefully balance these factors to achieve the desired heat transfer rate and efficiency.
Other Thermal Devices
Beyond heat exchangers, engineers also design and develop a wide range of other thermal devices, including:
- Boilers: Devices that generate steam or hot water for various industrial and residential applications.
- Condensers: Components that remove heat from a gas or vapor, converting it back into a liquid.
- Cooling towers: Structures used to dissipate heat from industrial processes or power plants into the atmosphere.
- Heat pumps: Systems that transfer heat from one location to another, typically for heating or cooling purposes.
Final Summary
Heat stands as a testament to the interconnectedness of the natural world. Its transformative power has shaped civilizations, driven scientific advancements, and continues to influence the very fabric of our existence. Understanding heat, its properties, and its applications empowers us to harness its potential for the betterment of society and the preservation of our planet.
Questions and Answers
What is the scientific definition of heat?
Heat is a form of energy that flows from a higher temperature region to a lower temperature region.
How is heat measured?
Heat is measured in units of calories or Joules.
What are the three modes of heat transfer?
The three modes of heat transfer are conduction, convection, and radiation.






Leave a Reply